Abstract
Objective: Pegylated liposomal doxorubicin (PLD) is a new class of anthracyclines widely used in hematological malignancies such as lymphoma and myeloma. Compared with traditional doxorubicin,PLD reduces the toxicity of doxorubicin to normal tissues, in particular the bone marrow and myocardium. PLD also reduces the occurrence of alopecia, increases the distribution of doxorubicin within the tumor, and can penetrate the blood-brain barrier and blood-testis barrier. In the 2013-2018 NCCN Guidelines, PAD regimens are listed as a class of recommended primary treatment regimens for transplant-eligible MM patients. The PDD regimen is listed as a category 1 recommended treatment regimen for patients with relapsed/refractory multiple myeloma. Data on the use of PLD in patients with newly diagnosed MM are sparse, and there are no comparable studies. This study mainly evaluated the safety and efficacy of PLD versus doxorubicin in the treatment of newly diagnosed multiple myeloma in the combination of bortezomib and dexamethasone.
Method: Newly diagnosed patients from 2015 in the above-mentioned hospitals were enrolled, aged >=18 years and <=70, with evaluable lesions, and ECOG 0-2. Patients were randomized to first receive a four-week PAD or PDD regimen as induction therapy, and the efficacy was evaluated after 4 courses of treatment. Afterwards, according to whether autologous hematopoietic stem cell transplantation was performed or not, they were divided into a transplantation group and a non-transplantation group (PAD/PDD continued treatment for 6 courses).Efficacy was reassessed after completion of treatment, and all patients subsequently received bortezomib monotherapy once per 2 weeks for 6 months. The primary endpoints were efficacy after induction and safety during induction therapy. Secondary endpoints were PFS and OS.
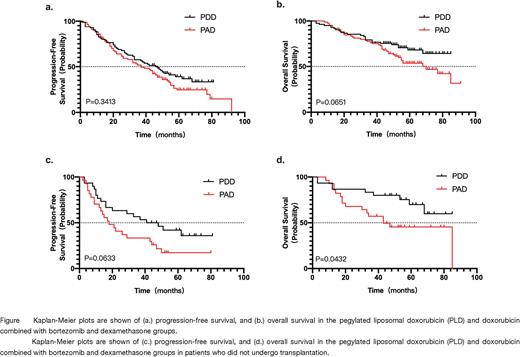
Result: A total of 183 patients were enrolled. 165 (90.16%) patients completed 4 courses of induction therapy. 107 (58.5%) patients completed autologous hematopoietic stem cell transplantation. The clinical characteristics of the patients are shown in Table 1.In the PAD group, 63.16% of patients had a VGPR and above, including 27.37% CR and sCR. In the PDD group, 69.32% of patients had a VGPR and above, and 29.55% of patients achieved CR or sCR(P=0.6141). Treatment-related mortality during induction was 0.54% (1 person), and grade 3-4 adverse events included infection (33.33%), thrombocytopenia (29.01%), neutropenia (25.93%), leukopenia (22.22%) and peripheral neuropathy (3.70%). The PAD group has more thrombocytopenia (grades 1-2 and 3-4), neutropenia (grades 3-4), leukopenia (grades 3-4), abnormal total bilirubin (grades 1-2) ), constipation (grade 1-2) than the PDD group (P values: 0.0009, <0.0001, 0.0001, <0.0001, 0.0341, 0.0014, respectively). With a median follow-up 51 months, the median PFS was 37 months, median OS was 77 months for all patients. There was no significant difference in PFS and OS between PAD group and PDD group. The PFS and OS of the transplantation group were significantly better than those of the non-transplanted group (median PFS 46 months versus 22 months, P=0.0002; the median OS not reached versus 53 months, P=0.0046). In the transplant group, there was no significant difference in PFS and OS between the PAD and the PDD induction. However, in the non-transplanted patient population, the PDD treatment had better PFS and OS than the PAD treatment (median PFS 44.5 months versus 18 months, P=0.0633; median OS not reached versus 43 months, P=0.0432).
Conclusion:In the MM induction regimen, the PDD regimen with 4 courses of pegylated liposomal doxorubicin instead of doxorubicin has a 29.6% CR above, 69.6% VGPR above. ORR was 85.3%, similar to PAD. The PDD regimen has less hematologic and hepatotoxicity. In transplantable patients, median PFS was 46 months, and OS was not reached. The 6-course PDD regimen had better PFS and OS than PAD in the non-transplant population. Therefore, replacing PAD with the PDD regimen of pegylated liposomal doxorubicin in the initial induction regimen is beneficial in both transplantable and non-transplantable MM patients.
Keywords: multiple myeloma, pegylated liposomal doxorubicin, doxorubicin, efficacy, adverse events, survival prognosis
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal